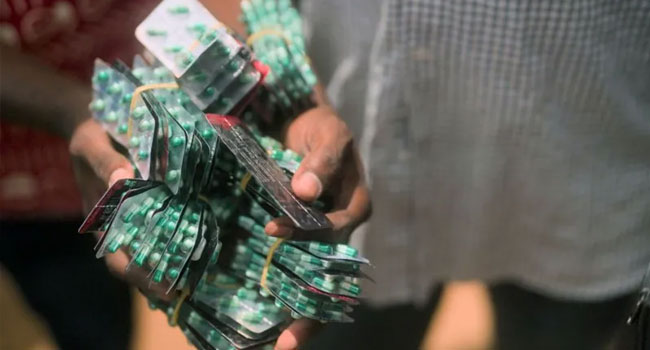
The National Agency for Food and Drug Administration and Control (NAFDAC) has blacklisted Aveo Pharmaceuticals Pvt Limited, an Indian pharmaceutical company, for allegedly producing and importing harmful opioid combinations into West Africa, including Nigeria.
In a statement on Friday, NAFDAC’s Director General, Prof. Mojisola Adeyeye, accused the company of manufacturing and distributing Tafrodol and Royal 225—drugs containing Tapentadol, a potent opioid, and Carisoprodol, a banned muscle relaxant. According to NAFDAC, these substances pose severe health risks, including respiratory failure, seizures, overdose, and death.
An investigation by the British Broadcasting Corporation (BBC) reportedly uncovered evidence linking Aveo Pharmaceuticals to the illegal exportation of these drugs. BBC’s findings revealed that packets of the drugs bearing the Aveo Pharmaceuticals logo were found on the streets of Nigeria, Ghana, and Côte d’Ivoire. Further evidence also confirmed that the company was involved in the illegal exportation of high-dose Tramadol, exceeding 100mg, which is unregistered and unapproved by NAFDAC.
Undercover footage reportedly captured Vinod Sharma, the company’s manager, admitting to distributing these opioids for street abuse across West Africa. In response, NAFDAC has banned the registration of Aveo Pharmaceuticals’ products in Nigeria.
The agency reaffirmed its commitment to protecting public health through stringent pharmaceutical regulations, including strict product registration, Good Manufacturing Practice inspections, post-marketing surveillance, and pre-shipment inspections for high-risk imports.








