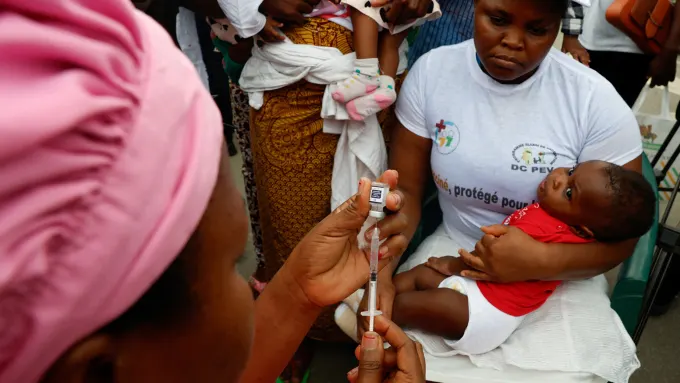
On Monday, July 15, 2024 children in Ivory Coast received the initial doses of the R21 malaria vaccine, a development praised as a significant advancement in combating one of the world’s deadliest diseases.
This vaccine, developed by the Jenner Institute at the University of Oxford and the Serum Institute of India (SII), is being distributed to several African nations, with South Sudan scheduled to begin vaccinations on Tuesday.
The vaccine is affordable, costing less than $4 per dose, and boasts an efficacy rate of 75%-80% in young children. The World Health Organization (WHO) projects that widespread implementation of the R21 vaccine, in conjunction with the RTS,S vaccine, could save up to 500,000 children’s lives annually.
Malaria, a preventable and treatable disease, caused around 608,000 deaths globally in 2022, with 95% of these occurring in Africa. The SII has already produced over 25 million doses and aims to manufacture up to 100 million doses annually to keep the vaccine affordable. Initially, 250,000 children under the age of 2 in Ivory Coast will be vaccinated, with Ghana, Nigeria, Burkina Faso, and the Central African Republic also authorizing the vaccine.
The R21 vaccine will complement the RTS,S vaccine, which has been administered to over 2 million children in Ghana, Kenya, and Malawi over a four-year pilot program, reducing all-cause mortality by 13%, according to UNICEF. Both vaccines have received WHO approval and are expected to significantly improve public health, alongside other preventive measures like mosquito nets.
The vaccination campaign involves a three-dose schedule, typically administered at five, six, and seven months of age, with a booster given a year later. Implementing this schedule in low-income countries will require substantial training and infrastructure development.